Carbon Filtration: Clearing the Air

Activated and Impregnated Carbon Media can be effective at filtering vapor contaminants out of the airstream by a process called adsorption. Carbon media is an effective adsorbent because it is a highly porous material and provides a significantly large surface area to which contaminants may adsorb. Air filtration by adsorption is the process many laboratories use to provide a safe and clean environment for their personnel. In order to maintain this safe and clean environment, filtration systems should be monitored, and the filters should be replaced when necessary. Therefore, it is critical to understand how long carbon filters should last, or at the very least, to understand when your filters become saturated, and need replacement.
What is my carbon filter's capacity?
Carbon filter capacity is expressed in several different ways, but two methods are most common.
-
Filter manufacturers typically list dedicated catalog numbers for filters of standard nominal sizes. A filter’s capacity is often expressed as a chemical-specific theoretical volume that it has the capacity to retain before saturation for that chemical. For example, a filter is rated to hold 250mLs of xylene, then the theoretical volume capacity of that filter for xylene is 250mLs. Whereas, the same filter may be rated to hold 60mLs of isopropyl alcohol, then the theoretical volume capacity of that filter for isopropyl alcohol is 60mLs.
-
Carbon substrate manufacturers and filter manufacturers may also choose to express a filter’s capacity for any given chemical by providing a filter-weight capacity percentage. This is a “capacity by weight” ratio that can be used to calculate chemical volume based on filter size. As an example, if a carbon filter has a filter-weight capacity for xylene of 27.6%, you could theoretically trap 27.6 grams of xylene in 100 grams of its carbon. This can be converted to volume by dividing the chemical weight by the chemical’s specific gravity (27.6 grams / 0.86 grams/mL = 32 mLs is retained on a 100 gram filter).
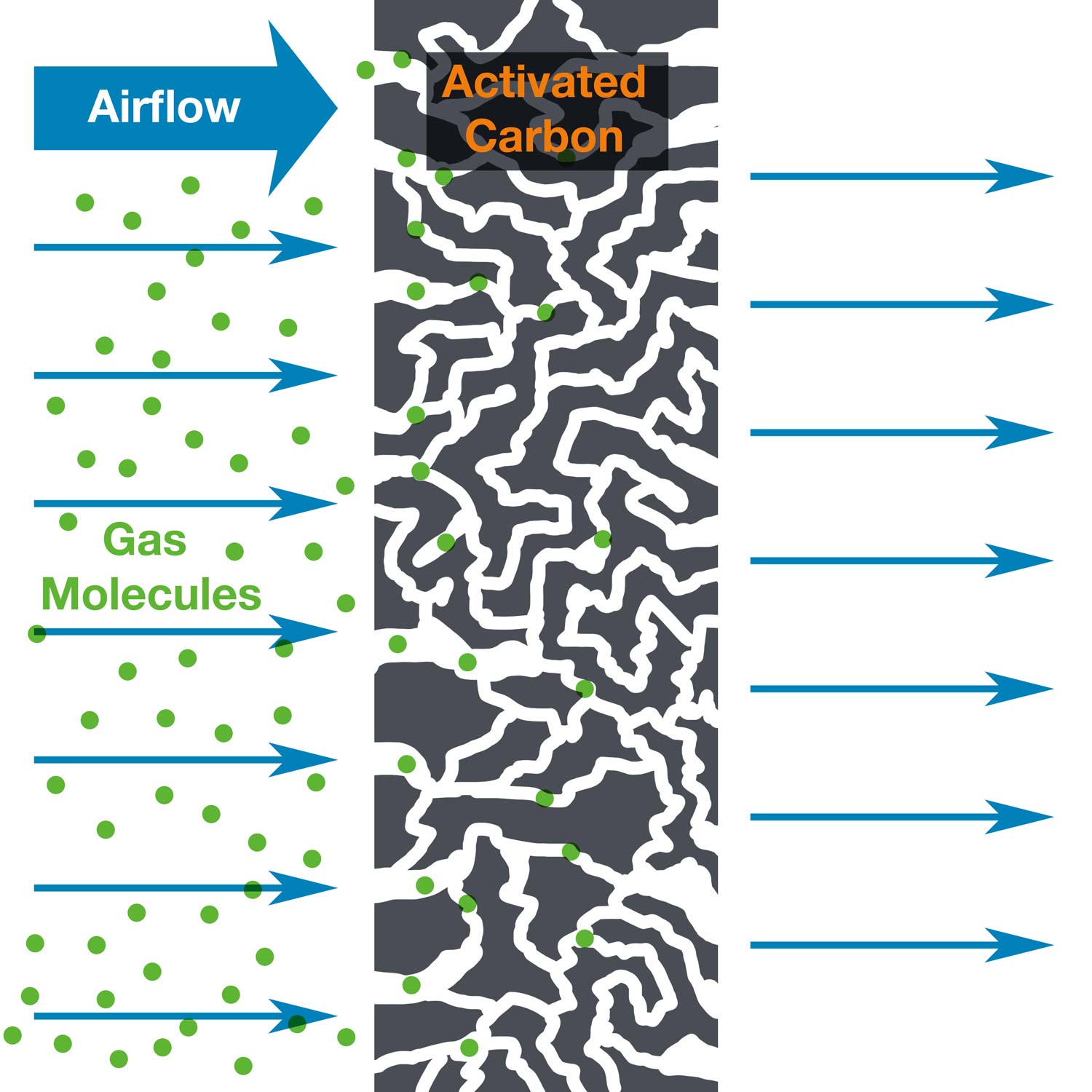
Are the capacities provided theoretical or realistic values?
Make sure you ask for clarification.
If you've spent time in a chemistry laboratory, you'll realize that the theoretical values obtained through stoichiometry and other calculations aren’t precisely yielded in the real world. Unpredictable variables can affect the actual results. Realistically, the filter capacity estimates are recommended to be ~1/3 of calculated theoretical values. This produces a safe estimate, to allow for variables that could negatively impact the estimated theoretical filter capacity.
What factors affect a chemical’s filtration efficiency?
Evaporative concentration, # of chemicals in use, molecular structure and molecular weight.
One major factor in filtration capacity revolves around the evaporative concentration of the given chemicals. Chemical adsorption is a result of the chemical concentration in the airstream attempting to reach equilibrium with the carbon media. The ultimate capacity of the activated carbon will increase significantly as the concentration in the airstream goes higher. Therefore, the higher the concentration, typically the better the yielded adsorption capacity.
But there is a catch - the higher the concentration, the higher the volume of chemical going into your filter. An increase in efficiency and capacity does not always mean an increase in how long your filters will last.
Here’s another fun, but not so wonderful, fact: working with mixtures causes a reduction in filter capacity by as much as 25-40%. Think of your filters as batteries. If Battery A is used to operate Device 1 (a television remote) until it is exhausted then it will last X minutes. If Battery A is used to operate Device 2 (a remote-controlled car) until it is exhausted then it will last Y minutes. Assuming that the car will drain Battery A faster than the TV remote, if Battery A is used to operate both devices at the same time until it is exhausted, the battery will last a shorter duration than it did when operating the device with the shorter battery life. For carbon filters this is 25-40% shorter.
Of course, there are exceptions to this rule. Some chemicals experience “displacement” during carbon adsorption. This happens when chemicals with varying affinities to the carbon media are in play.
Chemicals experience adsorption by making Carbon-to-Carbon (C=C) bonds with the carbon media. The greater number of C’s in its chemical structure, the greater number of C=C bonds the chemical can make with the carbon media, having a greater affinity to it. If a chemical that has a very weak affinity for activated carbon (such as methyl alcohol, with only 1 Carbon in its structure) is paired in an application with a chemical that has a higher affinity (like xylene, with 8 Carbons in its structure), then the xylene, with greater carbon affinity, will be trapped more effectively, and it can actually bump the methyl alcohol off of the carbon media, releasing it into your laboratory atmosphere.
Displacement may also happen if you are dealing with chemicals that have significant differences in their molecular weight, since the chemical’s molecular weight can also affect its affinity for the carbon media. Chemical adsorption is better for compounds with similar molecular weights and structures, therefore, since carbon media has a higher molecular weight, chemicals with higher molecular weights are more effectively adsorbed, again, resulting in displacement of the chemical with the lesser molecular weight.
If your displaced chemical has a low exposure limit or low toxicity threshold, use extreme caution, and monitor your filter’s status/exhaust airflow. You may need to employ a secondary filtration device, or use this chemical in a standard ducted enclosure.
These and other common variables, such as those listed below, and all factor into the life of a carbon filter:
- Specific Chemical characteristics (molecular weight, volatility, size, etc.)
- Chemical concentration
- Evaporation rate
- Volume of chemical released into the stream
- Humidity
- Temperature
- Presence of other chemicals
- Filter materials
- Chemical mixtures
With the appropriate information and chemical data, carbon filter life can be calculated through a simple set of formulas (Carbon Filter Capacity) for most chemicals.
When will I know my Carbon Filters are Saturated?
Automated Sensor or Manual Detection.
The estimated filter capacity is helpful information, but in order to maintain a safe laboratory environment it is critical to know when it is time to replace the filters. The two primary means of determining filter saturation are by manually monitoring the filters, or by relying on the system’s automatic filter saturation alarm to indicate saturation.
Manual filter monitoring is done with the use of chemical detector tubes and an air sampling device, which allows the user to test the exhaust air of the system using a chemical color indicator tube to determine if chemical breakthrough is occurring. Alternatively, many Carbon Filtration Systems do also have an automated Filter Saturation Alarm on them, which can audibly and visually alert the user when chemical filter breakthrough is occurring.
Is Your Application Suitable for Carbon Filtration?
Contact the Manufacturer.
Per SEFA 9 (Scientific Equipment & Furniture Association), your laboratory's chemical safety officer (CSO) should ask the manufacturer about the suitability of carbon filtration for their specific application. If changes are made in laboratory practice or the intended purpose of a carbon-filtered device, the laboratory should assess how the changes may affect this equipment and its performance. Contact one of our experts if advice is needed for filtering your chemical combination, or fill out our Chemical Assessment Form, and one of our experts will follow up with you in response.
Works Cited
"Carbon Filter Capacity: Calculate This!" Labconco General Chemistry eNewsletter August 2012.
Labconco Corporation. Chemical Guide for Carbon Filtered Enclosures. Kansas City, 2019.
Scientific Equipment & Furniture Association. SEFA 9: Recommended Practices for Ductless Enclosures. Garden City, NY, 2010.
| chevron_left | Online tool helps find all necessary fume hood installation components | Articles | The roles of lab planning | chevron_right |






